Abstract
While familial leukemia and bone marrow failure syndromes present typically in children, it is possible that less damaging variants, such as heterozygous alterations of recessive genes, may be identified in older patients with seemingly spontaneously acquired disease. This may be particularly true for recessive variants with lower penetrance. Gain-of-function germline (GL) mutations in SAMD9 have been described in the context of MIRAGE syndrome, characterized by Myelodysplasia Infection, Restriction of growth, Adrenal hypoplasia,Genital phenotypes and Enteropathy. Recently, pediatric myelodysplastic syndromes (MDS) families affected by a somatically acquired -7/del(7q) were found to have GL mutations in SAMD9 and SAMD9L on 7q21.2. Here, we focused on sporadic adult myeloid neoplasm patients to assess how frequently mutations in SAMD9 and SAMD9L occur with or without -7/del(7q).
We first sequenced a cohort of patients for the presence of variants in SAMD9/SAMD9L . Paired disease and normal GL DNAs were obtained from 312 MDS patients for whole exome sequencing (WES; n=220) and targeted deep sequencing (n=92). GL DNA was obtained from either buccal mucosa or paired CD3+ T cells. We used Broad Institute's Exome Aggregation Consortium (ExAC) data as ethnically matched healthy control (n=45,950).
We defined "rare variants" as those present in <1% of ethnically matched healthy controls. Among 312 patients with myeloid neoplasms, we identified a total of 30 rare GL variants of SAMD9/SAMD9L in 29 patients (9.3%). Validation was performed by Sanger sequencing or PCR amplicon sequencing. None of these alterations were present in any of the previously described familial cases. All patients had a heterozygous configuration except for one. We used an algorithm that predicts the pathogenic significance of SAMD9/SAMD9L alterations by evaluating conserved amino acid residues and alterations of protein features. We identified 18 (10 in SAMD9 and 8 in SAMD9L) which were most likely to be functionally deleterious; 7/19 in SAMD9 and 9/11 in SAMD9L were not previously described, emphasizing their rarity. For example, I268T and D550V of SAMD9 which are present only in MDS patients, and E220G, G235S, W333C and Y705C SAMD9L alterations, were predicted to be highly deleterious based on structure/function analyses according to prediction algorithms. While those with known population frequencies were not over-represented individually, their combined frequency was higher in MDS vs . ethnically matched healthy controls, with an odds ratio = 1.54 (95% CI: 1.04-2.27, P=.03), and frequencies of 29/312 (9.3% in MDS) vs . 2,896/45,950 (6.3% in healthy controls). Two patients had a family history of MDS and unexplained anemia.
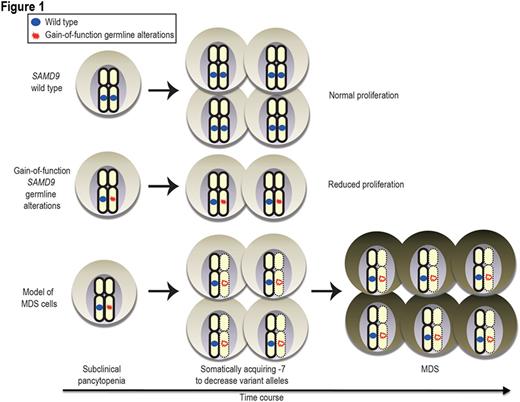
We then focused on patients with -7/del(7q). In total, 82 out of 312 patients (26%) had -7/del(7q); 5 of these 82 patients (6.1%) had SAMD9 alterations. Variant allele frequencies (VAFs) were significantly lower in unpurified bone marrow fractions than in GL controls in 3 out of 5 patients. For example, the GL of one patient had the S86F alteration in SAMD9 in a heterozygous configuration, whereas the tumor of the patient with somatically acquired -7 showed lower VAFs. This observation suggests that the SAMD9/SAMD9L variant allele was selectively deleted at the onset of MDS, resulting in retention of only the wild-type allele during clonal selection (Figure 1). SAMD9/SAMD9L can maintain cell growth by attenuating proliferation. SAMD9/SAMD9L GL mutations identified in familial MDS cases strongly repressed cell proliferation by working as gain-of-function mutations. Somatic acquisition of -7/del(7q) can rescue the growth-repressing effects from GL gain-of-function alterations. However, cells which lost the GL gain-of-function alterations by -7/del(7q) to gain a survival advantage progressed to MDS due to haploinsufficiency of chr7 genes. This interpretation is further supported by the observation of a lower frequency of variants in those with vs . without -7/del(7q) (5/82 (6.1%) in -7/del(7q) carriers vs. 24/230 (10.4%) in diploid cells). Our results support a model wherein SAMD9/SAMD9L variants are selectively lost in MDS with -7/del(7q).
In conclusion, while deletion of several important genes on chr7q are likely to be pathogenic in sporadic adult MDS, we show here that GL alterations in SAMD9/SAMD9L may also drive selection of -7/del(7q) clones in a small fraction of adult MDS.
Makishima: Yasuda Medical Foundation: Research Funding. Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal